Authors: Fabrizio D’Ascenzo, Ovidio De Filippo, Guglielmo Gallone, Gianluca Mittone, Marco Agostino Deriu, Mario Iannaccone, Albert Ariza-Solé, Christoph Liebetrau, Sergio Manzano-Fernández, Giorgio Quadri, Tim Kinnaird, Gianluca Campo, Jose Paulo Simao Henriques, James M Hughes, Alberto Dominguez-Rodriguez, Marco Aldinucci, Umberto Morbiducci, Giuseppe Patti, Sergio Raposeiras-Roubin, Emad Abu-Assi, Gaetano Maria De Ferrari
Commentary by: Fabrizio D’Ascenzo
Division of Cardiology, Cardiovascular and Thoracic Department, Città della Salute e della Scienza, Turin, Italy
Patients with acute coronary syndrome (ACS) are at high risk for ischaemic and bleeding events, with both being drivers of adverse prognosis. Careful evaluation of these risks plays a fundamental role in the clinical management of each patient, with important implications regarding the choice of optimal medical therapy for secondary prevention. To this aim, several predictive tools have been developed to estimate ischaemic and bleeding risks following an ACS, some of which have potential to support clinical decision making around the optimal duration of dual antiplatelet therapy (DAPT). However, the overall accuracy of these scores, along with their generalisability to external cohorts, remains modest, representing an unmet need for individualised patient management strategies.
Thus, to overcome the analytical limitations of current predictive tools, D’Ascenzo et al. adopted a machine learning-based approach to develop a risk stratification model to predict all-cause death, recurrent acute myocardial infarction, and major bleeding after ACS.
A dataset of 19 826 adult patients with ACS obtained from the observational BleeMACS and RENAMI intercontinental registries served as derivation cohort. A dataset of 3444 adult patients with ACS obtained from the European SECURITY randomised controlled trial, the FRASER registry, the Prospective Registry of Acute Coronary Syndromes in Ferrara and the Clinical Governance in Patients with ACS project of the Fondazione IRCSS Policlinico S Matteo served as the validation cohort.
The structured dataset included 25 variables: 16 clinical variables (age, sex, diabetes, hypertension, hyperlipidaemia, peripheral artery disease, estimated glomerular filtration rate [EGFR; using the Modification of Diet in Renal Disease study formula21], previous myocardial infarction, previous percutaneous coronary intervention, previous coronary artery bypass graft, previous stroke, previous bleeding, malignancy, ST-segment elevation myocardial infarction [STEMI] presentation, haemoglobin, and left ventricular ejection fraction [LVEF]), five therapeutic variables (treatment with β blockers, angiotensinconverting enzyme inhibitors or angiotensin-receptor blockers, statins, oral anticoagulation, and proton-pump inhibitors), two angiographic variables (multivessel disease and complete revascularisation), and two procedural variables (vascular access and percutaneous coronary intervention with drug-eluting stent).
Four machine learning models using different classifiers were developed to predict the occurrence of each of three outcomes: all-cause death, recurrent acute myocardial infarction, and major bleeding 1 year after discharge.
The derivation cohort was randomly split into two datasets: a training (80%) cohort, which was used to train the four machine learning models and tune their parameters, and an internal validation (20%) cohort, which was used to test the developed models on unseen data and to fine-tune the hyperparameters. The best-performing model for each study outcome (that resulted to be the adaptive boosting classifier for all the outcomes) was selected as the PRAISE model.
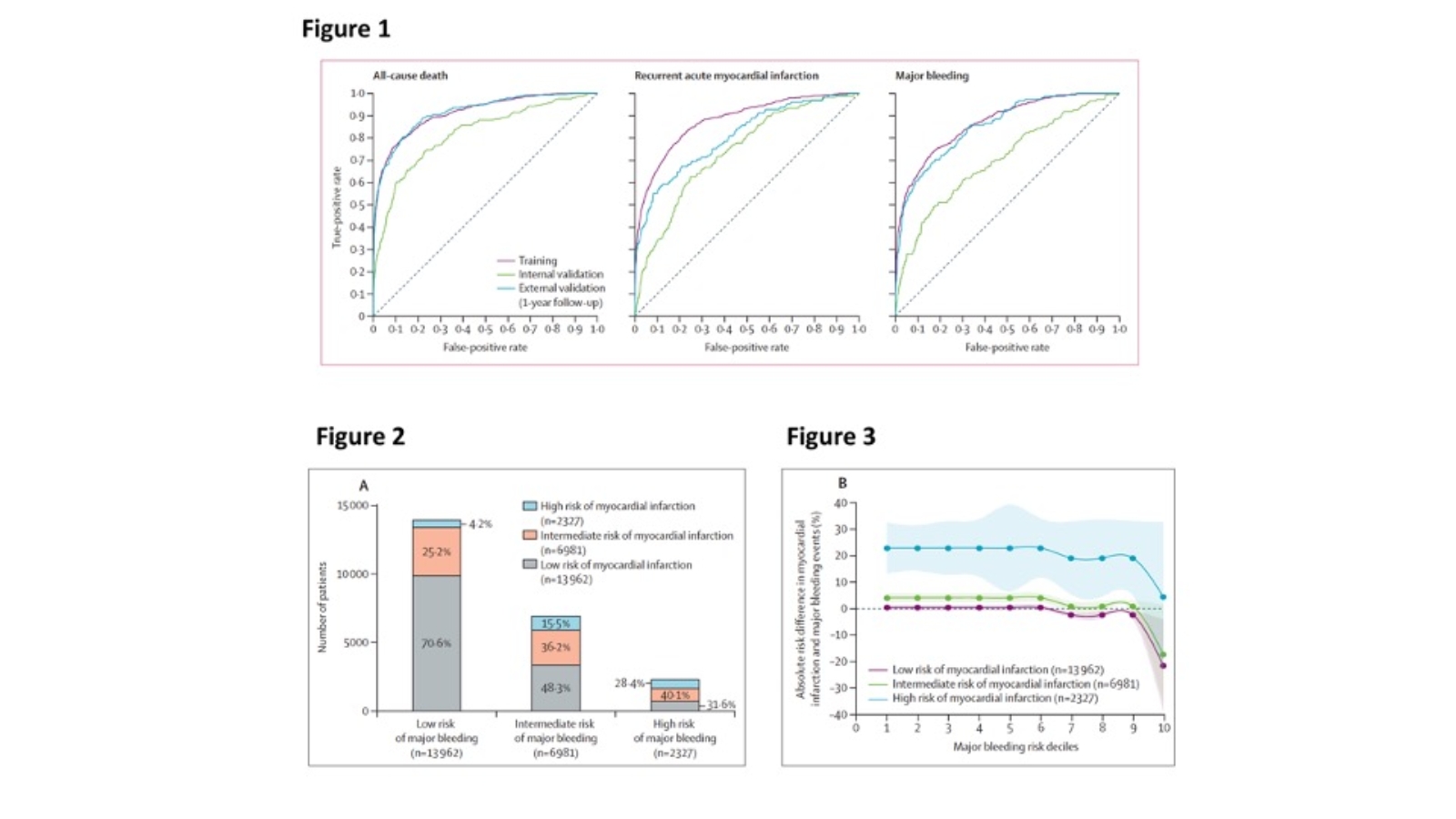
The PRAISE score showed an AUC of 0.82 (95% CI 0.78–0.85) in the internal validation cohort and 0.92 (0.90–0.93) in the external validation cohort for 1-year all-cause death; an AUC of 0.74 (0.70–0.78) in the internal validation cohort and 0.81 (0.76–0.85) in the external validation cohort for 1-year myocardial infarction; and an AUC of 0.70 (0.66–0.75) in the internal validation cohort and 0.86 (0.82–0.89) in the external validation cohort for 1-year major bleeding (Figure 1).
To translate the model data into clinically relevant information, the 23 270 patients of the pooled dataset were grouped into levels of low, intermediate, and high risk with thresholds reflecting clinically meaningful gradients in risk from one group to the next, and cross-classification of the pooled cohorts with myocardial infarction and major bleeding risk scores was determined (i.e., classes were established by combining the risk categories of the myocardial infarction and major bleeding PRAISE scores, Figure 2). The hypothetical trade-off between ischaemic and bleeding risks for each patient was assessed by plotting the absolute observed risk difference between myocardial infarction and major bleeding in each of the nine risk classes against the predicted myocardial infarction and major bleeding risk in each class (Figure 3). Among patients classified as being at high risk of myocardial infarction, the absolute observed risk difference between myocardial infarction and major bleeding events supported a consistent prevailing ischaemic risk regardless of the major bleeding PRAISE risk class (with the exception of the highest PRAISE bleeding risk decile). For patients classified as being at low risk of myocardial infarction, their risk of major bleeding exceeded their risk of myocardial infarction once they were classified as being at intermediate-to-high risk of major bleeding.
In summary, the PRAISE scores presented excellent discriminative abilities for the prediction of 1-year all-cause death, myocardial infarction, and major bleeding following an ACS, also when externally validated. Clinically meaningful risk cutoffs for all-cause death, myocardial infarction, and major bleeding PRAISE scores would classify 60% of patients with ACS as being at low risk (<1% probability) of post-discharge ischaemic and bleeding events 1 year after discharge, and 10% of patients with ACS as being at high risk (>19%) of these events. Moreover, robust hypothetical trade-offs in the occurrence of ischaemic and bleeding events were observed for each patient according to their myocardical infarction and major bleeding score classes, that may in theory allow for DAPT tailored treatment, if prospectively studied and validated in a randomized fashion.
In conclusion, this study showed that a machine learning-based approach in the setting of post-ACS risk prediction is feasible and effective with potentially important implications on the optimization of the quality of care.
Read More: https://pubmed.ncbi.nlm.nih.gov/33453782/